Cyclopropyl Methyl Bromide is a chemical compound known for its usefulness in the field of organic synthesis. In this blog post, we'll explore the properties of this compound, its applications, safety considerations, and its role in various chemical processes.
Overview
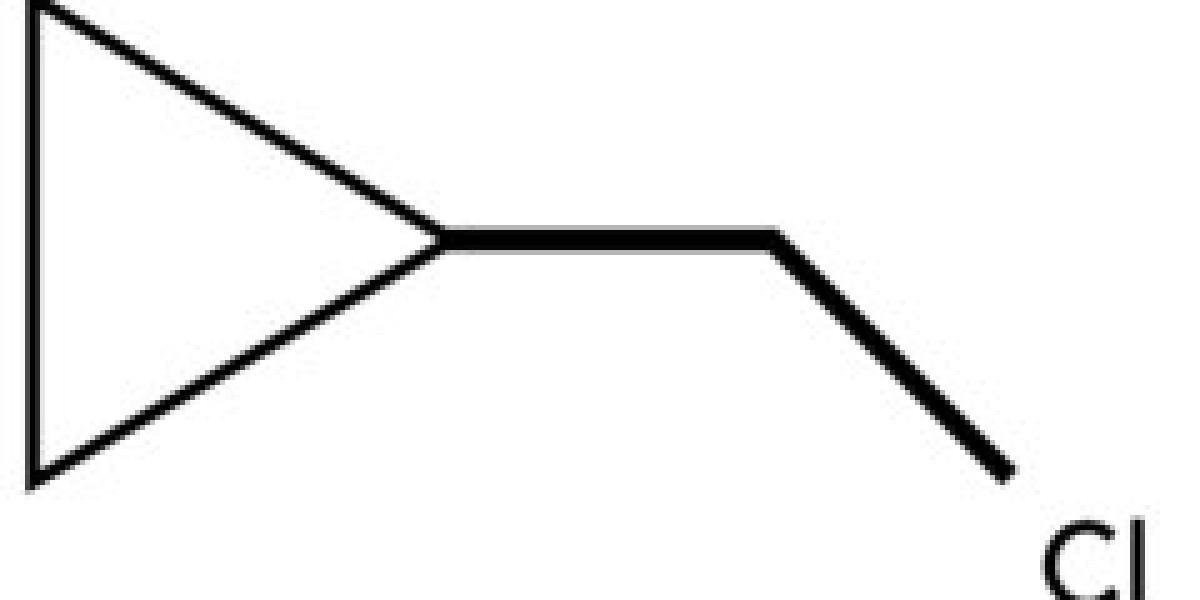
Cyclopropyl methyl bromide, also known as 1-bromo-2-methylcyclopropane, is an alkyl bromide compound that features a cyclopropane ring structure and a methyl group attached to a bromine atom. Its molecular formula is C4H7Br, and its structural formula can be visualized as a three-membered cyclopropane ring with a methyl group and a bromine atom attached to one of the carbons.
Physical and Chemical Properties
- Appearance: Cyclopropyl methyl bromide is a colorless liquid.
- Odor: It may have a pungent odor.
- Boiling Point: The boiling point of cyclopropyl methyl bromide is around 82°C.
- Density: Its density is about 1.4 g/mL.
- Reactivity: Cyclopropyl methyl bromide is a reactive compound due to the presence of the strained cyclopropane ring and the bromine atom. It can undergo various chemical reactions, such as nucleophilic substitution.
Applications
Cyclopropyl methyl bromide is primarily used in organic synthesis for the following purposes:
- Alkylating Agent: It can transfer the cyclopropyl or methyl group to other molecules, thereby serving as an effective alkylating agent.
- Synthesis of Pharmaceuticals: This compound can be used in the synthesis of certain pharmaceuticals and other complex organic molecules.
- Material Synthesis: It may also be used in the preparation of specialty chemicals and materials.
Safety Considerations
While cyclopropyl methyl bromide is a useful compound, it is important to handle it with caution due to its potential hazards:
- Toxicity: It can be toxic if ingested, inhaled, or absorbed through the skin.
- Irritation: It can cause irritation to the skin, eyes, and respiratory system.
- Reactivity: It should be stored away from heat, flame, and incompatible substances due to its reactive nature.
- Protective Measures: Proper safety gear such as gloves, goggles, and lab coats should be worn when working with this compound.
Handling and Storage
When working with cyclopropyl methyl bromide:
- Ventilation: Ensure proper ventilation in the workspace.
- Storage: Store the compound in a cool, dry, and well-ventilated area, away from light and incompatible substances.
- Disposal: Dispose of any waste material according to local regulations and guidelines.
Conclusion
Cyclopropyl methyl bromide is a valuable compound in the realm of organic synthesis, offering opportunities for creating new molecules and materials. However, its reactivity and potential hazards require careful handling and appropriate safety measures. By following best practices, researchers and chemists can harness its potential safely and effectively.